Remdesivir V Covid-19: New data supports it as a treatment
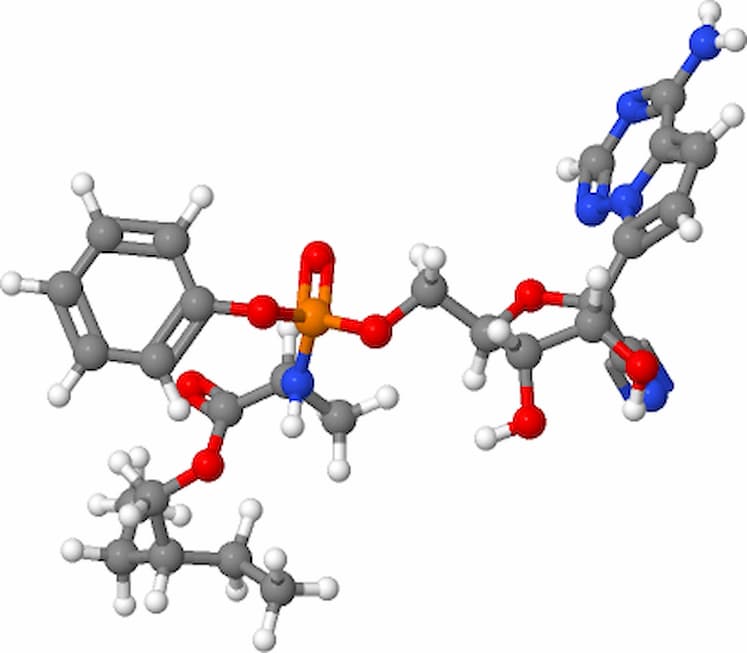
Rhesus macaques with COVID-19 that were given Gilead Sciences\’ remdesivir on the same infusion schedule as that being tested in human clinical trials saw their symptoms improve, researchers reported.
Gilead Sciences’ COVID-19 hopeful remdesivir has kicked up plenty of controversy as the company speeds up R&D amid questions about whether early human trials of the drug have adequately proven it works. Now, scientists at the National Institutes of Health and Gilead have published results from an animal trial of remdesivir that they believe will help the scientific community better assess the drug’s power to combat the virus.
In a trial involving 12 rhesus macaques, early treatment of COVID-19 with remdesivir reduced symptoms of the disease and lung damage, they reported in a study posted on bioRxiv. Six macaques were treated with the drug, while the others were untreated. Seven days after the start of the study, the macaques treated with remdesivir were significantly healthier than those in the control group, according to the study (PDF).
The animal trial was designed to replicate the remdesivir dosing schedule that’s being tested in the ongoing clinical trial. So the macaques received an intravenous dose of the drug 12 hours after being infected with COVID-19, followed by one booster dose per day for six days. At the end of the week, all six untreated animals were showing breathing difficulties. Only one of the treated macaques had breathing problems, which were mild, they reported.
The Sanford Burnham Prebys-led team started by screening a library of 12,000 existing compounds that have been shown to be safe for use in people. They searched specifically for drugs that could halt the replication of the virus that causes COVID-19, SARS-CoV-2.
They found 30 drugs that were able to prevent the virus from copying itself—including remdesivir and the antimalarial chloroquine derivatives that have also generated interest during the pandemic. They suggested that the additional four options they uncovered should also enter human trials. That’s because they all work differently than remdesivir and the antimalarials do, and their safety profiles have been well characterized already, they said.
“Many drugs identified in this study—most of which are new to the COVID-19 research community—can begin clinical trials immediately or in a few months after additional testing,” said senior author Sumit Chanda, Ph.D., director of the Immunity and Pathogenesis Program at Sanford Burnham Prebys, in a statement.
Courtesy of FierceBiotech by Arlene Weintraub
You might also like
For relevant updates on Emergency Services news and events, subscribe to EmergencyServices.ie