AstraZeneca vaccine to be restricted for use with Irish people over 60
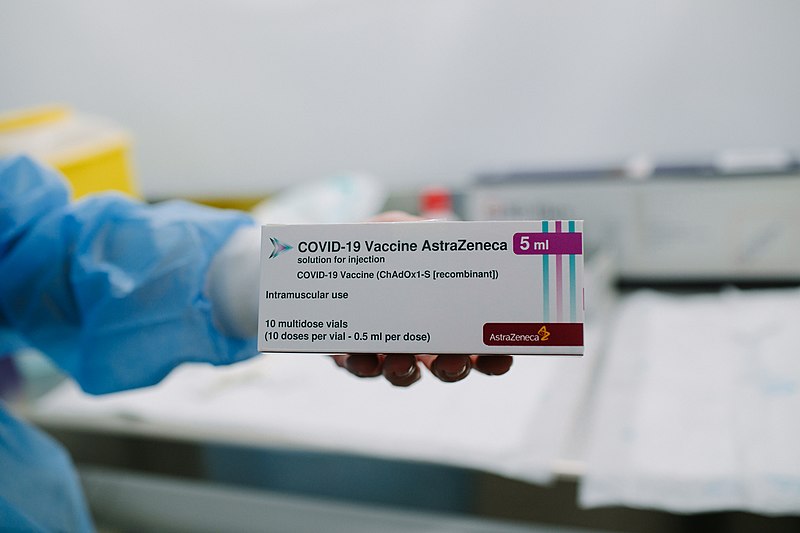
Reports say it will be a precautionary recommendation as questions still being asked on safety in older patients
The expert committee advising the government on vaccines is expected to recommend restricted use of the AstraZeneca vaccine in the over 60s in Ireland.
A formal announcement is expected later this evening, following a long meeting of the National Immunisation Advisory Committee today, RTE news is reporting.
NIAC experts gathered today to look at the latest evidence in relation to the side affects of the AZ vaccine in older people, following restrictions being placed on its use in several European countries.
NIAC is now expected to recommend to the Health Minister Stephen Donnelly that a pause or significant restrictions are placed on the roll-out of this one vaccine in older people in Ireland pending further research and information.
Dr Ronan Glynn, deputy Chief Medical Officer, and the Department of Health will be formally informed of the recommendations, which are being seen as a precautionary measure for the moment.
There have been reports of a possible link between the vaccine and very rare blood clotting issues in a small number of adults who have been given the AZ jab.
It is not known yet what impact the decision will have on the wider national roll-out of the vaccine programme.
Earlier today, the HSE said they expect that 180,000 people will be vaccinated against the coronavirus this week.
A growing number of EU countries have introduced age restrictions on the use of the AZ vaccine.
Last week, the European Medicines Agency released its findings on a possible link between the AZ vaccine and blood clotting.
In Ireland, over 233,700 doses of the AstraZeneca vaccine have now been administered and the programme is expected to continue as planned for those under 60.
Source: www.corkbeo.ie
You might also like
For relevant updates on Emergency Services news and events, subscribe to EmergencyServices.ie